A Batch Record is a document that provides the complete manufacturing history of a pharmaceutical product. It aims to assure the safety and quality of the manufactured product by:
- Providing processing instructions to the operator during the execution of manufacturing processes
- Documenting the manufacturing process exactly as executed.
A Batch Record, sometimes also called a Batch Manufacturing Record (BMR) or a Batch Production Record (BPR) is a batch-specific copy of a Master Batch Record. The Master Batch Record can be seen as the blueprint of the process, while the Batch Record contains the documentation of one single execution of the process.
You can get more info about batch tracking system at https://factory-talk.com/it-solutions/electronic-batch-record-ebr.
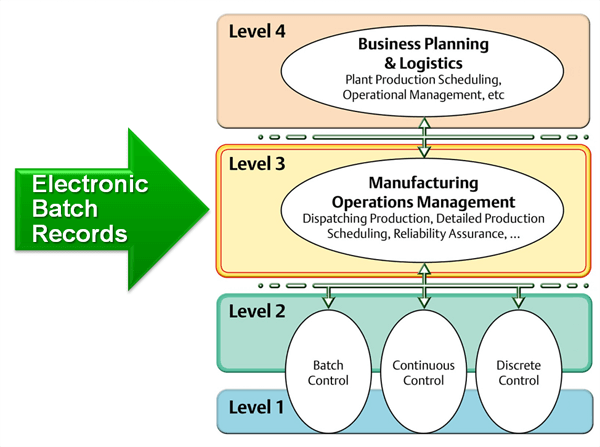
It explains exactly how a lot of a product is manufactured and provides info on who, when, where, and how the different process steps are executed. It also contains all the in-process and release tests that assure the quality of the lot and documents any deviation from the standard process.
In 2018, 49% of the warning letters sent out by the FDA included a data integrity component . As the Batch Record is the crown witness of the quality of your product, it is no surprise that the data integrity and the traceability of every component in the document is of the utmost importance. An often-used set of principles that define the integrity of a record is “ALCOA” (see table).
There are obvious differences in how paper-based records manage these principles compared to electronic systems.
Paper-based batch records might for example require you to manually copy data such as lot numbers and expiry dates, or they might require on the spot calculations for a dilution or a cell count. On a digital record, these tedious and error-prone tasks can be replaced by a barcode scanner, picture proofs and automated calculations.